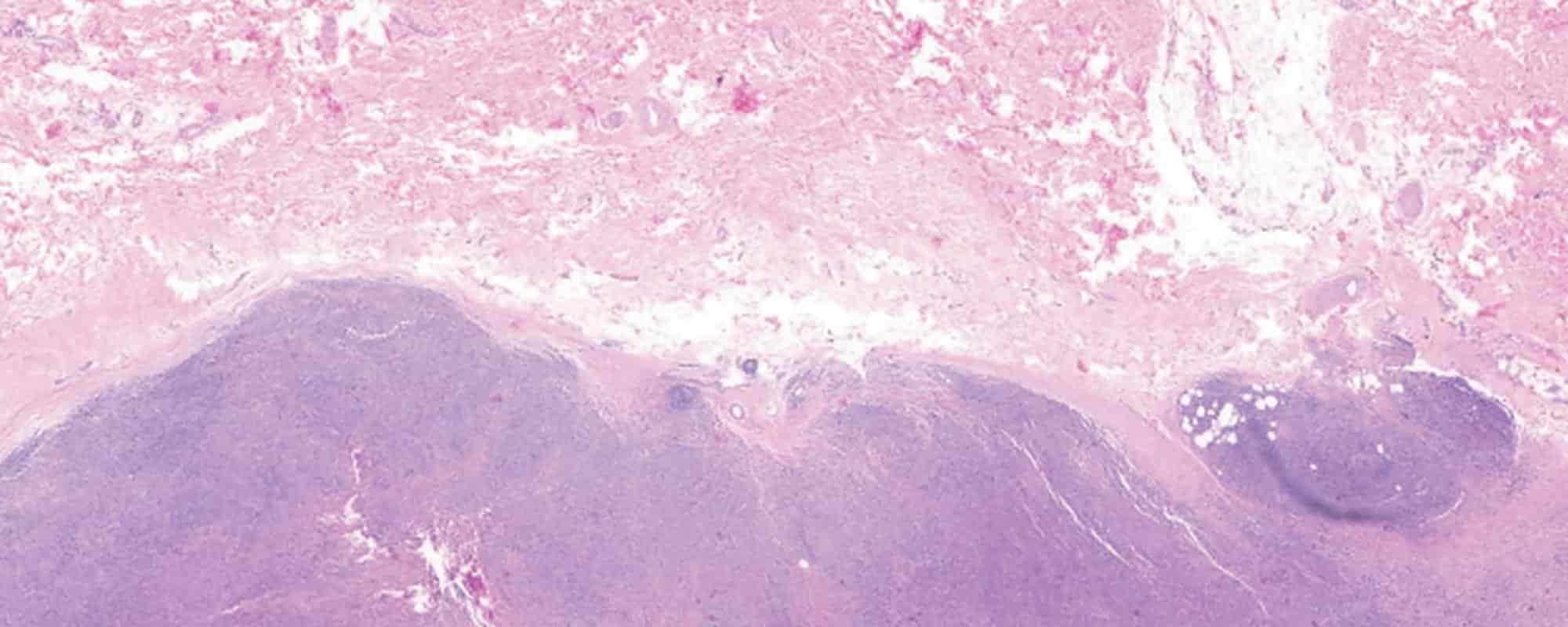
Cancer behaviour is highly complex. Tumour cells are adept at evading the body’s defence mechanisms and therapeutic drugs over time. At Mater Dei Hospital, a multidisciplinary team of health professionals is analysing a rare case of cancer cell transformation that may uncover new therapeutic approaches for patients with this cancer type.
Continue readingDesigning a Dream
Can we reduce the level of pain perception in the brain? Distraction therapy has been used by health professionals for years to help children and young people cope with painful procedures. The aim is to take the focus of the patient away from the pain and concentrate on something else instead. Books, games, music, and toys are some of the many types of distraction therapy.
Continue readingPutting patients first
Most cancer treatments involve complex surgeries, toxic drugs, or taxing radiation, but there are other answers to this devastating disease. Prof. Pierre Schembri-Wismayer is developing a vaccine that works by harnessing our body’s own immune response and directing it towards the threat, fighting the disease as an inside job. Words by Gail Sant.

Our bodies produce billions of cells every day. With such industrial production rates, it’s entirely likely that a mistake or two are made along the way. Cancer cells are those mistakes—faulty mutants.
Humans are also equipped with mechanisms that allow them to recognise cancer cells and get rid of them, but there can be trouble when distinguishing ‘bad’ from ‘good’. Part and parcel of cancer is that it compromises the immune system to ‘escape’ our ‘guards’. This precious time during which the body fails to recognise the mutants is the golden opportunity for cancer cells to multiply and thrive. And as the cancer grows, so do the problems that come with it.
Despite the varied types of cancers in existence, treatment usually entails surgery and chemotherapy. But the risks and side-effects that come with them are heart-wrenching.
But what if a vaccine can stop cancer in its tracks? Prof. Pierre Schembri-Wismayer and his team are working on a type of immunotherapy that enables the body to recognise the invading cancer sooner, directing the attack as a result.
How it works
Vaccines work by triggering an immune response in the body. These cancer vaccines developed by Schembri-Wismayer work the same way. He collects a piece of the cancerous tumour and denatures (a process not unlike cooking or boiling) it in formalin, which modifies its shape, making it easier for the body to recognise as a foreign body. The body can then remove it.
Our bodies have a naturally low tolerance when it comes to foreign entities, so when a vaccine injects the cooked tumour, the body recognises it and ambushes both the injected and the original cancer present in the patient, ‘engendering a stronger immune response’.
Inspired by a Japanese research paper, yet baffled by its lack of recognition, Schembri Wismayer modified the method outlined there and created his own version of the vaccine to accommodate his first patients: a pair of pet rats.
The founding pets
Schembri-Wismayer had the perfect opportunity to test the potential cancer treatment when a student’s pet rats fell ill. Their owner mistakenly overfed the rats to such an extent that they became obese. ‘The rats became square-shaped,’ Schembri-Wismayer notes. With the increase in body fat, their oestrogen rose too—a female sex hormone that increases the risk of breast cancer. As a result, both rats developed the disease, and even showed metastasis in both underarms, which also happens in humans. Obesity is linked to cancer in many animals.
Within days, Schembri-Wismayer took samples from the rats and produced a tailor-made vaccine for each rat. Two weeks after their treatment, the rats’ owner informed him that they were in a lot of pain. ‘But tumours don’t hurt,’ Schembri-Wismayer explains.
An operation on the rats revealed that the tumours had broken down (were full of dead cells). This confirmed that the rats’ pain was actually stemming from the inflammation caused by the vaccine itself.
Physical anguish aside, this was a good sign, an indication that the body was fighting back. In the end, the tumours burst, necrosed, and died. The rats beat the cancer, and thus a new research project found its beginnings.

Hurdles ahead
Having had such promising results, and with ethical approval from the local Animal Welfare Council as a therapeutic option, Schembri-Wismayer turned his attention to a group of animals which would benefit a lot from such vaccines—people’s pets.
‘In many cases, once a dog or a cat gets cancer, there’s not much you can do if it spreads,’ says Schembri-Wismayer. His vaccines can offer new hope to pet owners when cancer strikes and the only option is to put them down. And so it has.
With consent from owners and vets, Schembri-Wismayer offered the therapy to cats and dogs of different breeds. The types of cancer varied, as were their progressions, and so the results were just as jumbled. The treatment was successful for some, but not all. Considering this treatment is still in its early days, an element of trial-and-error puts some animals at a disadvantage, particularly those who are very ill when the disease is in its last stages. It should also be mentioned that this research project was held back by challenging communication difficulties between everyone involved. ‘Different priorities made the process more difficult than it had to be,’ Schembri-Wismayer notes. The ideal scenario would see him and the veterinarian working hand-in-hand to follow up on the animals and their response to the treatment using blood tests and ultrasound.
That said, the potential of the treatment isn’t limited to individual successes. Each pet-patient contributed to a better understanding of the treatment, especially when owners were immensely helpful and allowed the veterinary surgeon to provide the team with a piece of the tumour after treatment (even if the pet was put down). With that in mind, the prospects of this type of immunotherapy are promising to say the least.
Moving onto human trials
While the vaccine’s promising results might be a step in the right direction, the cure for cancer doesn’t seem to be in the near future. The ongoing animal treatments are providing useful information, but the move to human trials is gruelling. Testing out new therapies comes with storey-high hurdles, including financial ones, that need to be overcome.
The reality is that this kind of therapy would work best as a first response (or after debulking surgery). Because the more widespread the cancer, the bigger the immune response the vaccines create. And ‘if a reaction is strong enough, it might be enough to kill the patient,’ Schembri-Wismayer explains. But medicine works with a set of rules and best practices. Doctors are obliged to try what’s known to work first before moving onto lesser-known experimental drugs. This fact is a challenge. Despite the hurdle, Schembri-Wismayer is certain that people would eventually volunteer to be involved in clinical trials, as happened with HIV/AIDS treatments. ‘Unfortunately in end-stage cancer you have no other options.’
Vaccines for human use need to be produced in Good Medical Practice (GMP) facilities, cites Schembri-Wismayer. However, there are no such facilities locally. After a long search, he has finally found an industry partner that has agreed to create the vaccines in Belgium, against a price, as long as he sets up the clinical trials. This little victory comes with its own set of problems. In this case, the new limiting factor is mainly funding. Clinical trials typically cost millions.
The politics of Cancer
Logistics aside, the hunt for a cure faces problems even more complex than what already seems immensely problematic. Schembri-Wismayer explained that there’s a major systematic flaw.
‘Many cancer researchers are not doctors. Their career depends on peer-reviewed publications and not finding a cure.’ This means that as long as their findings are statistically significant or are ‘good enough’ to be published in a high-end scientific journal, their job is done.
Granted, no research finding can be seen as wasted knowledge. Each can be seen as a small step forward. However, Schembri-Wismayer believes that the millions of funds designated to cancer research should have more rapid deliverables that directly benefit the patients, not just academic careers. Unfortunately, the cancer research community is a circular one, where scientists review other scientists mainly based on publications to get further funding.
‘I am not trying to publish in Nature [the world’s top academic publication], I’m trying to cure cancer’, says Schembri-Wismayer as he confesses that he feels guilty about not letting his students publish papers. But this is a necessary evil in the journey towards therapies. ‘Once a method is published, no company will touch it because it’s in the public domain.’ The method would have no monetary value since anyone could copy it. Needless to say, no pharmaceutical company will industrialise a drug that doesn’t guarantee profit.
Speaking of profit, Schembri Wismayer expressed that the financial aspect of this study is one of his greatest motivations. ‘Most of these drugs cost the Earth’, he said, adding that ‘when each shot costs €30,000 and the success rates are low, very few National Health Care systems are going to provide it.’ Take Yervoy, a drug commonly used to treat melanoma; one dose can cost up to €4,000. Survival rates after treatment are low. And there are no ‘money-back-guarantees’ if the outcome is less than satisfactory. As a result, studies have shown that a quarter of cancer patients in the US choose not to take such prescriptions because of such high prices. Money shouldn’t be the limiting factor in the fight for survival. Schembri-Wismayer believes in cancer treatment that’s affordable for everyone.
Fighting the good fight
Even in the face of all these odds, Schembri-Wismayer persists. A cancer patient isn’t just a number; a cancer patient is also a mother, a brother, or a friend. And knowing this is enough to help him and thousands of other cancer researchers to continue pushing through.
One in two people in the UK will be diagnosed with cancer during their lives. It is a harsh reality, but humankind will eventually find a cure just as it has with other previously deadly diseases such as influenza or measles. This vaccine is in its early stages but with the proper support, it may contribute to an affordable, life-saving cure.
Written in blood
Maltese researchers are leading the way in developing new diagnostic tools for cancer. Dawn Gillies finds out more from Prof. Godfrey Grech and Dr Shawn Baldacchino.
Breast cancer survival rates have been improving steadily in recent years. In Malta, 86.9% of patients currently survive, up 7% over the last decade. Thanks to new targeted therapies, the outlook is increasingly bright. But precision therapies need precision testing.
Breast cancer diagnosis has reached new heights and with current tests using tissue biopsies, pathologists can classify patients for specific treatment. Precision medicine goes a step further. It provides more information, predicting the aggressiveness of the cancer and measuring the number of cells from the tumour that spread into the bloodstream.

This does not mean that all requirements in precision therapy have been met.
At the time of writing, there is no simple method to test patients’ ongoing benefit from treatment or to measure different tumour areas from one sample. For this to be possible, we need super-sensitive tests. This is where Prof. Godfrey Grech and Dr Shawn Baldacchino at the University of Malta come in.
Detecting the undetectable
During his PhD, Baldacchino studied a new class of breast cancer representing most cases of the triple negative type, which affects 12% of breast cancer patients in Malta.
In triple negative breast cancers, tests for estrogen receptors, progesterone receptors, and excess HER2 protein all result in negatives and are associated with aggressive tumours.
To detect this new class of breast cancer, Grech’s team have created a new test that uses molecular substances we naturally produce in our body—biomarkers. By pinpointing the right combination of certain biomarkers, they can test for this new class within the triple negative breast cancer cases.
They initially used the test to look at biopsies from past patients. These exercises showed that they could accurately detect the cases—even in samples that were over a decade old! In fact, the test was so successful that the team is now working with biological testing industry giant Luminex to use it in hospitals worldwide. With a patent filed, research labs will get their hands on it later this year with the hope that by 2021 it will be used to directly help patients in hospitals.
However, there is more work ahead. Encouraged by the results so far, the team wants to take the test and other current biomarker tests a step further. They want to use a simple blood sample which is less invasive, allowing patients to be monitored during therapy.
Pushing boundaries
With the method Grech and his team have optimised, obtaining information on new classes of patients that predict therapy use, detecting different tumour areas in one sample, and the use of blood to monitor the benefits of therapy have become a

Photo by James Moffett
possible reality. With technologies from Luminex and Thermo Fisher, they can now read over 40 biomarkers in one test simultaneously. But with blood they need a new angle. And that is happening through another test using particles that originate from cells called exosomes.
Exosomes are tiny messenger bubbles which cells release into the blood . ‘We believe that when there is a tumour in the patient, there will be a signature in these exosomes circulating in the blood,’ says Baldacchino.
Finding these exosomes could mean detecting cancer at an earlier stage than is currently possible. The team believes they would be able to detect the exosomes that point to cancer long before a tumour shows up in scans and other regular tests—and so, they would be able to nip the cancer in the bud. But to do this, they need to be able to decode the messages the exosomes are carrying.
Positives for patients
It’s not only in the realm of breast cancer diagnosis and classification that the team can help patients—they might also be able to improve treatment. ‘Most targeted therapies currently try to inhibit specific receptors and proteins to stop the uncontrolled growth of cancer cells,’ Grech says. But through their research, the team has found that targeting the low activity of specific complexes of proteins in tumour cells is key. Their research models show that increasing the activity of these protein complexes is possible using specific drugs.
This is true for triple negative breast cancer, where the amount of PP2A protein is extremely low. The PP2A protein enables the body to fight the cancer, so increasing its activity would create a chain reaction in the body which could limit the growth and spread of that category of cancer cells.
This approach to treatment has applications beyond triple negative breast cancer. Grech is hopeful that PP2A production could be amped up for different types of cancer too, and lead to positive results.
Managing the unmanageable

When organising a project like this, it’s expected that things won’t go to plan. One of the biggest challenges for Grech’s team has been establishing collaborations with other groups across the globe. They need these connections to provide the samples required to test their systems. With other groups working on similar projects, time is a limited resource. Thankfully, the team found collaborators in Leeds (UK), and Barcelona (Spain), allowing the group access to the samples they need.
What is certain is that support for this work has come in many shapes and forms. The project received funding both from public donations and the Malta Council for Science and Technology. Baldacchino also found an ally in the charity foundation Alive with the help of the Research Trust of the University of Malta (RIDT). He is the first recipient of funding from them, and their first graduate.
Predicting the future
Thanks to projects like these, cancer research has a bright future in Malta. The team has their product launch to look forward to later this year, which will see a drastic reduction to the time and effort it takes researchers and doctors to determine the type of breast tumour.
But a lot of challenges lie ahead. The biggest challenge will come in the move to early stage cancers. These cancers have low levels of substances to detect, which means that any test they develop will have to be extremely sensitive in order to be effective. Successfully identifying these cancers would signal a massive breakthrough for the global medical community—and, more importantly, for patients. Early detection through basic blood tests would open the door to early stage treatment and a higher rate of survival. Nothing could matter more.
Project ‘Accurate Cancer Screening Tests‘ financed by the Malta Council for Science & Technology through FUSION: The R&I Technology Development Programme 2016.
Aspirin and Cancer
Aspirin is often considered a wonder drug due to its versatile use in treating inflammation, reducing pain, and helping to prevent heart-related disease. However, there is more to it. Aspirin is actually cancer-preventive. Studies have shown that a daily low dose of aspirin, medically prescribed for more than five years, lowers the risk of cancer-related deaths by at least 30%. So, should we all start taking aspirin on a daily basis to lower our chances of getting cancer?
No, not exactly. This is because many aspects of aspirin’s cancer-preventive effects are still poorly understood. Particularly, researchers have not yet pinpointed what enables aspirin to selectively kill early-stage cancer cells and not healthy cells. This is the scope of the research currently being carried out at the Yeast Molecular Biology and Biotechnology Laboratory (headed by Prof. Rena Balzan).

The secret behind aspirin’s tendency to kill certain cells but not others seems to lie in the physiology of the exposed cells. Aspirin exploits the natural differences between healthy and cancerous cells to eliminate malignant cells before they can take over.
Oxygen, if transformed into ‘Reactive Oxygen Species’, is known to cause DNA mutations that can lead to cancer. Through this research, we studied mutated yeast cells which are a relevant model of early-stage cancer cells due to their low tolerance to oxygen-associated stress. We then identified genes in these mutant yeasts which are affected by aspirin.
One of aspirin’s targets is a key metabolite required for the production of energy-rich compounds vital for cell survival. We found that aspirin creates a shortage of this metabolite in mutated yeast cells, causing them to run out of energy and die.
This implies that early-stage human cancer cells may suffer a similar fate and, more importantly, partly explains how aspirin prevents tumour formation. Such knowledge may prove useful in the development of novel anti-cancer treatments.
This research was carried out as part of Project “R&I-2015-001”, financed by the Malta Council for Science & Technology through the R&I Technology Development Programme. This research is being carried out as part of Azzopardi’s Ph.D. project at the Centre for Molecular Medicine & Biobanking and the Department of Physiology & Biochemistry, University of Malta
Author: Maria Azzopardi
Luminex xMAP®: Enhanced lab efficiency
Stereotypical depictions of researchers involve crazy hair, oversized goggles, shabby lab coats, and loads of test tubes. While the first three may be exaggerated, the sheer volume of tubes and wells needed in a lab cannot be overstated, especially when the lab is dedicated to anything biological.
One tissue sample can be used for a gamut of tests, all of them attempting to identify something different in it, be they antibodies, DNA, or RNA (biomarkers). Often, many samples are required due to all the tests needed to highlight the variations in those biomarkers. But the size of samples is now decreasing thanks to machines like the Luminex System running xMAP technology.
The Luminex System is a research/clinical diagnostics platform that allows detection of multiple analytes in a single well of a microtiter plate—100 or more reactions using a single drop of fluid.
Multiplex assays are widely used in experiments investigating the characteristics of molecules within a biological sample. This approach can be used to see whether an experimental treatment works, or what changes a DNA mutation causes in the molecules or molecular pathways within cells.
In real terms, this machine allows for analyses to be done to determine whether or not a patient has a particular disease or gene variant in their blood that would prevent a drug from being effective. It also allows them to determine the ideal dosage for those drugs. The machine can also be used to identify and characterise viral infections.
A particular research group at the University of Malta, headed by Prof. Godfrey Grech, has used Luminex xMAP technology to develop novel markers which are allowing them to classify a subset of triple-negative breast cancer
patients.
By identifying these biomarkers, it may be possible in future to detect the disease earlier and give patients better-targeted therapy.

Author: Prof. Godfrey Grech
Malta’s brightest exports: Travelling to the EU’s JRC
A group of Maltese researchers travel to the European Commission’s Joint Research Centre site in Ispra to share their work in the fields of climate change, environment, and medicine. Cassi Camilleri writes.
Leaving a legacy
A legacy gift is a planned future donation to a charity or trust which is given through a will or other designation. It allows individuals to express personal values by integrating charitable, family, and financial goals. Planned gifts drawn up in wills can be made in cash, or by donating assets such as stocks, real estate, and art pieces. The possibilities are endless.Continue reading