Colouring Chemistry
Smart, logical, and colourful. Dr David Magri and his team develop intelligent molecules. They are not only smart because they perform logical functions, similar to computers, but because they can detect miniscule changes in their surroundings that causes them to emit a large array of colours. These colour changes are intriguing researchers and are a great benefit to society.
Labs in solution
Imagine the smallest thing you possibly can. The eye of a needle? A human hair? A particle of dust? Think smaller, something you cannot even see, something on a molecular scale. Now imagine that molecule has the potential of a whole laboratory. This dream is now becoming a reality.
In recent years, the field of molecular sensors has grown into one of the most ground-breaking areas in Chemistry. Molecular sensors are compounds that can detect a substance, or unique mixture of substances, and provide an easily detectable output. Usually this is a change in the absorption of ultraviolet or visible light, or in the emission of Fluorescence. In other words: colours!
John Gabarretta (supervised by Dr David Magri) created a simple example of these fluorescent molecular sensors. The molecule was based on the Fluorophore-Spacer-Receptor model, where the ‘output’ part of the molecule (the fluorophore — a structure which shines light) is separated from the ‘input’ part (the receptor — a structure which is sensitive to a particular substance, such as acidity or a metal ion) by an intermediate spacer, whose main function is to link these two components together. The model means that a molecule can detect a chemical and respond by shining light or not. The process gives information about the chemicals in a solution.
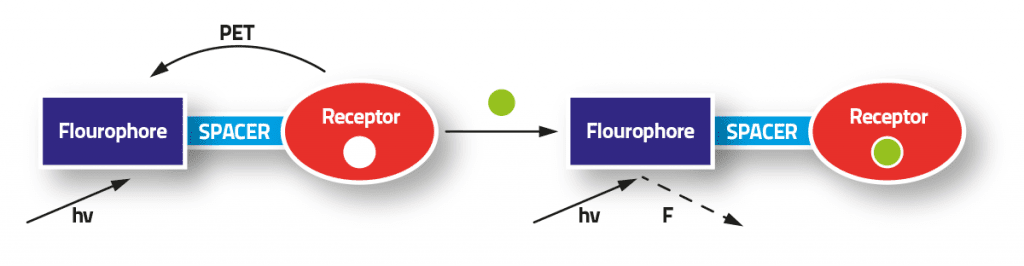
The molecule was made by a two-step synthetic route (which took several attempts and resulted in several different colours), and its behaviour was tested by dipping into an acid. In water the molecule was switched ‘off’, but quickly turned ‘on’ in an acidic solution by giving a bright blue light when exposed to ultraviolet light (UV) — a pretty satisfying sight!
Molecular sensors have some very advanced applications — the pioneer A. P. de Silva said that there is room for a “small space odyssey with luminescent molecules”. This odyssey includes some that detect substances such as sugars.
While very advanced systems are approaching chemical computers, since they have multiple inputs and use Boolean Logic, the so-called ‘Moleculator’ or ‘gaming tic-tac-toe’ systems. The future is bright (if you pardon the pun) and with more complex structures more possibilities will appear; the molecular laboratory may become a reality detecting diseases or toxins in no time at all.
This research was performed as part of a Bachelor of Science (Honours) at the Faculty of Science.