To defeat your enemy, you must understand your enemy. We can’t confirm if Sun Tzu would agree with this, but the MPitNET group at the University of Malta (UM) certainly does. THINK dives into their efforts in understanding – and hopefully defeating – pituitary tumours.
I was enjoying lunch with a recently made friend when they casually dropped ‘I have cancer.’ With a sunny disposition, quick laughter, and a seemingly healthy appearance, my friend’s news came as a shock to me. I have been living closely with cancer patients for almost a decade, and I have witnessed the debilitating side effects of treatments. In my head, this friend didn’t fit the profile of a cancer patient, but years of scientific research has allowed them to live an almost normal life, practically unburdened by the debilitating side effects of other cancer therapies. For the first time, I saw cancer as a chronic, manageable disease instead of a dark cloud of slowly leaking time, hovering over the patient’s head.
It is towards these life-changing advancements that the MPitNET team, led by principal investigators Prof. David Saliba (Associate Professor of Applied Biomedical Science at the Faculty of Health Sciences) and Prof. Josanne Vassallo (Professor of Medicine at the Faculty of Medicine and Surgery and Consultant Endocrinologist at Mater Dei Hospital), is working. The group of researchers at UM work with pituitary tumours, which develop in the ‘master’ gland called the pituitary gland at the base of the brain. Although these tumours are usually benign (meaning that they don’t invade other tissues), they can wreak havoc, causing dysregulation of hormone secretion and even loss of sight if the tumour size increases and presses on the optic nerves.
As highlighted by Vassallo, current therapies can control symptoms in a significant proportion of patients but only cure less than 40% of cases. This necessitates life-long follow up for recurrence. To this end, understanding the tumour microenvironment will enable scientists to find potential targets for therapy and improve patients’ quality of life and survival rates.
The MPitNET project entails two branches of research:
- Identification and mapping of the immune cells that infiltrate pituitary tumours
- Characterising the immune gene expression in the tumour microenvironment
This type of research can be referred to as ‘basic science’. The unassuming name hides the gigantic impact that it has. Although without direct clinical applications, ‘basic research’ is the foundation of many clinical studies. It forms the basis of biology from which clinical research can grow. In this case, MPitNET is looking for therapeutic targets, which include meaningful components of the cell machinery that can be modulated to attack and kill tumour cells. As Vassallo explains, the team is looking to understand ‘what makes the tumours happen, what happens in the cells surrounding tumours and use the information to find targets for therapy.’

Fighting the Rebels
So what exactly is the MPitNET group doing? Let’s start with identifying and mapping the immune cells that infiltrate pituitary tumours.
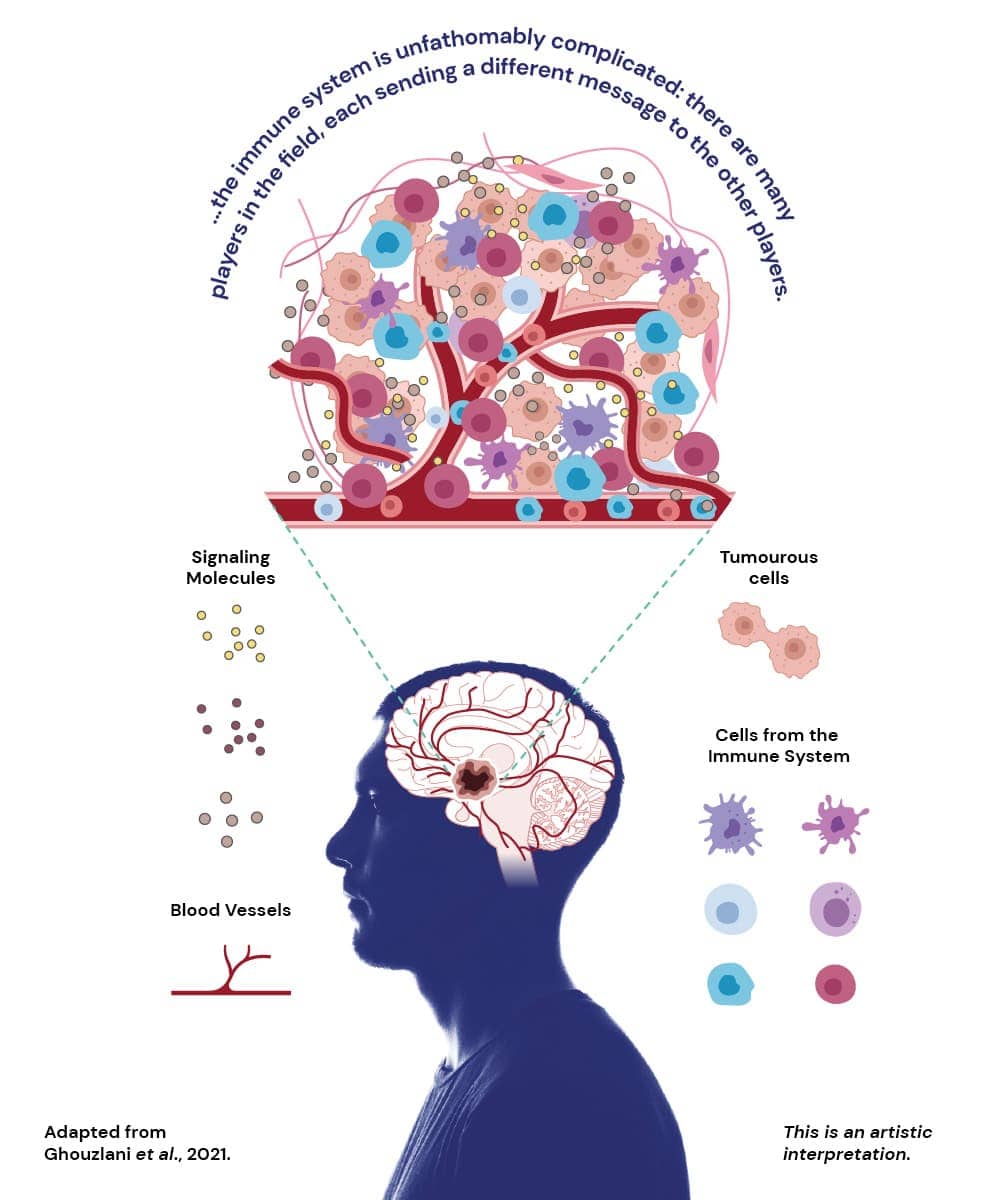
Cancer is a complicated beast. A tumour is composed of mutated cells that start growing uncontrollably. Simultaneously, our immune system, the defenders against invaders and rebels, can detect and attack tumour cells. But some of the mutated cells manage to evade these defence mechanisms. As the detectable tumour cells are killed, the sneaky cells continue dividing, indifferent to the efforts of the immune system. Time comes when the tumour only contains these evasive cells, and impervious to our body’s attacks, it continues growing, potentially invading other tissues.
Immunotherapy is a type of cancer treatment that tries to boost the patient’s immune system to identify and fight cancer cells. It tinkers with the signal between the different cells, making sure that the right message passes through: kill the rebel.
But the immune system is unfathomably complicated; there are many players in the field, each sending different messages to the other players. At the same time, the cancerous cells send their own signals and silence some of the immune system’s messages. Because every type of tumour is different, studying the immune environment of each tumour and understanding the signals being sent is essential to design therapies that work for each case.
MPitNET is studying which immune cells surround or infiltrate pituitary tumours. Besides identifying the type of cells, the team is looking at their location within the tumour and the messages they send via proteins called cytokines. By understanding how the immune system behaves around these tumours, they might identify therapeutic targets to eliminate those pesky tumours.
So far, Dr Oriana Mazzitelli (Research Support Officer with the Faculty of Health Sciences) has revealed a new population of immune cells present in all the tumours analysed with the capacity of killing tumour cells. It is the first time that these types of cells were observed in this context. Further studies will be done to validate these observations and assert the clinical relevance of these immune cells. Characterising the ratio of the population in different tumours may hint towards the prognosis of the disease and may also guide clinicians when choosing the best therapy for each case of pituitary tumours.
Excitingly, this novel population of immune cells may be present in other types of tumours, shining a new light into the play between the immune system and cancer cells.
To Read or not to Read
One of the components driving the triumph of cancer cells over the host tissue is their genes. Genes are the instruction manual of our cells; they tell the cell what to do and what its function is. So if a normal cell starts ‘reading’ (usually called expressing) genes that allow it to replicate indefinitely or evade the immune system from attack, then that normal cell can become a ‘bad’ tumour cell.
One of the theories for cancer genesis focuses on cells that start expressing genes which alter their function. These cells begin replicating uncontrollably, skipping the usual control steps that regulate cell division, growth, and differentiation. The quick, unregulated division can, in turn, generate new mutations that allow the tumour to keep evading the mechanisms set up to destroy it (be it the immune system or the therapies developed).
There are two ways for a cell to start expressing ‘tumorous’ genes (called oncogenes). It can have a mutation within the gene (the cell’s instruction manual is different from the cells that surround it), or instead, the cell can start expressing a gene that is not generally expressed in normal cells (it is dormant, silent).
Seeing the different gene expression between cells allows us to understand what a cancerous cell is doing differently. This will also show us the pathways and functions that we need to target to kill or modulate the cell.
Besides studying the relation between pituitary tumours and the immune cells, Ms Emma Jayne Spiteri set out to study the gene expression of the tumorous cells. She analysed tissue samples and isolated six genes that are expressed differently in the tumour’s surrounding environment, all linked to the immune system.
The team found out that different types of pituitary tumours had different expression levels of each one of the six highlighted genes. A gene usually instructs the cell to build a protein. If pituitary tumour cells are producing different proteins, then these could be driving its growth and could be possible targets for future therapies. Furthermore, if different tumours produce different proteins, researchers may be able to develop therapies targeting specific tumours, according to the proteins that they are expressing.
Next steps
The MPitNET team are continuing their work to further understand what is happening in these types of tumours. They now want to increase sample sizes, a crucial step to prove that their findings are the rule, not the exception. Because the techniques Mazzitelli is using need to be carried out on fresh tissue, she can only analyse the tumours right after they are excised from a patient. Considering that pituitary tumours are rare and only around 12 cases per year will require surgery, the number of fresh samples is low indeed. The team shall implement new techniques that allow analysis on frozen tissue stored in the repository. One of the techniques, immunohistochemistry, allows the researchers to determine the location of the immune cells in or around the tumour and will also help confirm the presence and location of the proteins coded by the genes she identified.
In parallel, the relation between the different immune populations that surround the tumour environment will be analysed. By confirming what immune cells are flocking to the tumours and what proteins are being produced inside the cells, the group will be able to determine if currently available cancer therapies can be applied to pituitary tumours. The knowledge could also help the development of new therapies in future, targeting the immune cells and proteins identified.
Although exciting, Vassallo warns that it might take decades for such therapies to become available to patients. ‘There is a certain impatience when someone reads about a potential new therapy for tumours,’ she explains. It is important to understand that research findings take time to test and validate, not to mention actually developing the therapies, carrying out trials, and finally using them to treat patients.
These advancements take time; they require thorough testing and continuous validation.
MPitNET’s work may not result in therapeutic advancements that will be used by my friend, but maybe one day, they can help others dissipate cancer’s dark cloud.
This project is being funded by the Emanuele Cancer Research Foundation https://ecrfmalta.com/
Further Reading
Ghouzlani A, Kandoussi S, Tall M, Reddy KP, Rafii S, Badou A. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Front Immunol. 2021 Jul 9;12:679425. doi: 10.3389/fimmu.2021.679425. PMID: 34305910; PMCID: PMC8301219.





Comments are closed for this article!