Written by Maria Cardona
Atmospheric carbon dioxide (CO2) levels have increased dramatically in the last few decades. Famous for causing global warming, CO2 is also resulting in the acidification of seas and oceans. This disturbs the rich life of the marine ecosystem, which affects human communities dependent on this environment for their livelihood. For islands like Malta and Gozo, this problem is particularly important. This ‘silent crisis’ has attracted the X-prize Competition organisers who have set a $2 million dollar prize to be awarded to anyone that can develop stable, inexpensive, and precise acidity (pH) sensors to help understand the acidification of marine environments. At the same time, a European COST initiative (Supramolecular Chemistry in Water) is encouraging the design of water-soluble molecules which can recognise analytes. Most chemical sensors do not perform well in water.
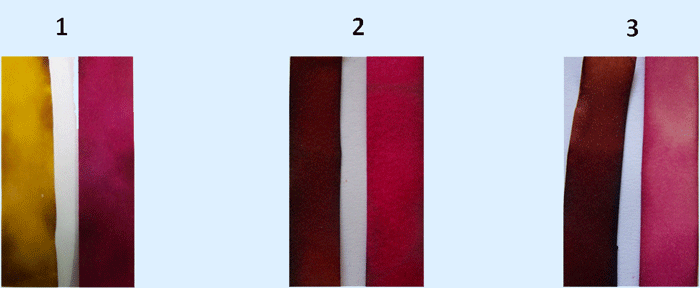
As a step to solve this problem, Maria Cardona (supervised by Dr David C. Magri) developed a number of water-soluble indicators that monitor pH levels by changing colour. The change is easily visible. The pH is a measure of the acidity or basicity of a solution. The indicators were synthesised in the lab using standard synthetic techniques.
The colorimetric pH indicators are based on the dye azobenzene and show brilliant and distinct colour changes with transitions between high to mild acidity, pH 1 and 4 (pictured). The molecules are water-soluble due to the incorporation of sulfonate groups onto the azobenzene-based molecules. A sulfonate group is a charged entity consisting of sulfur and three oxygen atoms. By its very nature, a sulfonate group is very polar and makes molecules more water-soluble. This contrasts with commercial azobenzene-based compounds such as methyl yellow and methyl red that have no charge and are not so soluble. The indicators’ structure and mechanism were further studied using a number of spectroscopic techniques to understand how they work.
The three azobenzene-based pH indicators are very brightly coloured. This class of compounds is widely used as colorants for food and cosmetics. Pending further tests to show non-toxicity, the azobenzenes could be used in common applications. Though a number of azobenzenes have been banned from use in edible products, the synthesized molecules are promising to be much safer because of the presence of the two sulfonate groups. They allow the molecules to be quickly and safely eliminated from the body.
The synthesis and study of readily soluble pH indicators is one approach to developing pH indicators to monitor the acidity of seas and oceans. By incorporating sulfonate groups, molecules can be rendered soluble in water. This is a significant contribution towards the detection of acidity in water.




Comments are closed for this article!